Lesson 17
Volume and Density
17.1: A Kilogram by Any Other Name (5 minutes)
Warm-up
The purpose of this warm-up is to get students to think more about what they mean by “light” and “heavy” to prepare for later activities that explore density.
Launch
Arrange students in groups of 2. After quiet work time, ask students to compare their responses to their partner’s and decide if they are both correct, even if they are different. Follow with a whole-class discussion.
Student Facing


Which has more mass, a thousand kilograms of feathers or a thousand kilograms of steel? Explain your reasoning.
Student Response
For access, consult one of our IM Certified Partners.
Activity Synthesis
Select students to share reasons that each might have more mass. It may be helpful to discuss how mass is measured to conclude that each, by definition, is the same mass. Then ask students to discuss what it means, specifically, when we say that feathers are lighter than steel, and how much volume a thousand kilograms of each substance might occupy.
Ask students to add this definition to their reference charts as you add it to the class reference chart:
The density of a substance is the mass of the substance per unit volume. That is, \(\text{density}=\frac{\text{mass}}{\text{volume}}\). (Definition)
density: 1 gram per cm3
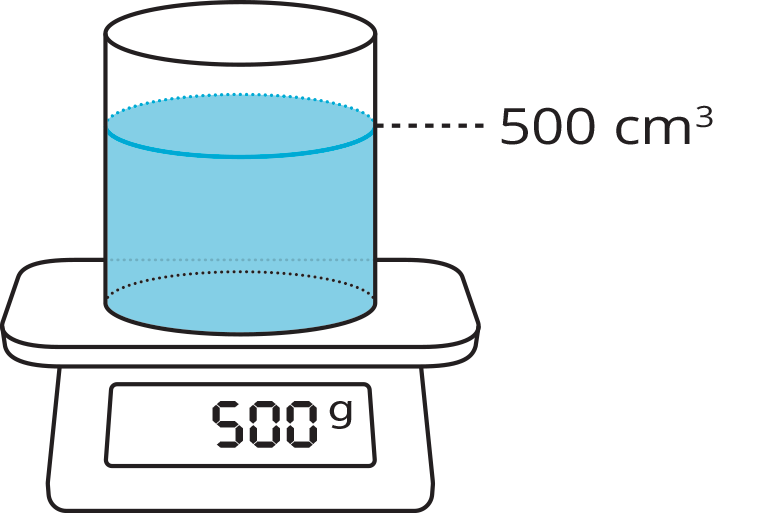
For example, a metal object whose mass is 150 kilograms with volume 1000 cubic centimeters has a density of \(\frac{150}{1000}\) or 0.15 kilograms per cubic centimeter. Each cubic centimeter of the metal contains 0.15 kilograms of mass.
17.2: Light as a Feather (15 minutes)
Activity
Students use concepts of volume and unit conversion to enhance their understanding of density.
Launch
Tell students that 1 cubic meter is equal to 1,000,000 cubic centimeters and 1 kilogram is equal to 1,000 grams. Suggest that students pay careful attention to units as they work through this task.
Monitor for students who calculate the feather density in grams per cubic centimeter then convert to kilograms per cubic meter, and those who begin the task by converting the measurements to kilograms and cubic meters.
Design Principle(s): Cultivate conversation
Supports accessibility for: Memory; Conceptual processing
Student Facing
The feathers in a pillow have a total mass of 59 grams. The pillow is in the shape of a rectangular prism measuring 51 cm by 66 cm by 7 cm.
A steel anchor is shaped like a square pyramid. Each side of the base measures 20 cm, and its height is 28 cm. The anchor’s mass is 30 kg.
- What’s the density of feathers in kilograms per cubic meter?
- What’s the density of steel in kilograms per cubic meter?
- What’s the volume of 1,000 kg of feathers in cubic meters?
- What’s the volume of 1,000 kg of steel in cubic meters?
Student Response
For access, consult one of our IM Certified Partners.
Student Facing
Are you ready for more?
Iridium is one of the densest metals. How many times heavier would a standard pencil be if it were made out of iridium instead of wood?
Student Response
For access, consult one of our IM Certified Partners.
Anticipated Misconceptions
Students may calculate density in grams per cm3, then be unsure how to convert to kg per m3. Prompt them to either convert the measurements to cubic meters and kilograms prior to calculating density, or to use dimensional analysis to convert the density.
Activity Synthesis
The purpose of the discussion is to draw out relationships between mass, volume, and density. Ask students:
- “How did you deal with the different units in this problem?” (If possible, select a student who calculated the feather density in grams per cm3 then converted to kg per m3, and another who converted the measurements to kilograms and cubic meters prior to calculating the density.)
- “How did you calculate the densities of each material?” (Divided the mass by the volume.)
- “How much space is 400 cubic meters? Would the feathers fill this room?” (A classroom of 30 feet by 30 feet by 12 feet has a volume of about 300 cubic meters.)
- “How much space is 0.124 cubic meters? Would the steel fit in the bed of a pickup truck?” (1,000 kg of steel would make a cube with edge length about 0.5 meters.)
17.3: A Fishy Situation (15 minutes)
Activity
This task presents a different way to think about density. Instead of considering mass per unit volume, students analyze animal population density. They use unit conversion and volume calculations to solve a problem. As students choose and track common units of measurement, they are attending to precision (MP6).
While students work, monitor for a variety of strategies such as:
- converting the density of 16 fish per 100 gallons of water to 0.16 fish per 1 gallon
- multiplying the tank’s volume in gallons by 16, then dividing by 100
- calculating that if 275 fish were used, the density would be about 14 fish per 100 gallons
Launch
Tell students that there are 7.48 gallons of water in 1 cubic foot.
Consider showing students pictures of the 82-foot tall cylindrical aquarium at the Radisson Blu hotel in Berlin, Germany.
Design Principle(s): Support sense-making
Supports accessibility for: Memory; Organization
Student Facing
An aquarium manager drew a blueprint for a cylindrical fish tank. The tank has a vertical tube in the middle in which visitors can stand and view the fish.

The best average density for the species of fish that will go in the tank is 16 fish per 100 gallons of water. This provides enough room for the fish to swim while making sure that there are plenty of fish for people to see.
The aquarium has 275 fish available to put in the tank. Is this the right number of fish for the tank? If not, how many fish should be added or removed? Explain your reasoning.
Student Response
For access, consult one of our IM Certified Partners.
Activity Synthesis
The goal of the discussion is to highlight different ways to solve the problem. Ask students what the density of fish per 100 gallons would be if 275 fish were put in the tank, and what that means in this situation. Invite students to share how they approached rounding. For example, if the calculations show that 315.8 fish are needed, should we round up or down? Both answers can be supported.
Lesson Synthesis
Lesson Synthesis
In this lesson, students used mass, volume, and density to solve problems. Here are some questions for discussion:
- “What are some things with very high density or very low density that you encounter in the world?” (Bowling balls, bricks, and certain metals are very dense. Wood has medium density. Styrofoam is not very dense. Air and other gases have very low density compared to solid objects.)
- “How can you tell if something is more or less dense than air?” (Things that are more dense than air naturally fall, but things that are less dense than air naturally rise, like helium or hot air balloons.)
- “What other kinds of density could there be?” (Any sort of measurement per unit of volume can be interpreted as density. For example, food could have a calorie density, like calories per serving. Even more abstractly, density can be interpreted as any ratio of measurements. For example, cost per square foot is a kind of density. Another example is the number of people per square mile in a city, which is called population density.)
17.4: Cool-down - Float or Sink? (5 minutes)
Cool-Down
For access, consult one of our IM Certified Partners.
Student Lesson Summary
Student Facing
Imagine you have a baseball and an apple the size of a baseball. If we weigh each, we’ll likely find that even though they’re the same size, the baseball weighs more.
A baseball has volume 200 cubic centimeters and weighs 145 grams, while an apple the same volume might weigh about 100 grams. We say that the baseball is more dense than the apple because it has more mass packed into each unit of volume. The density of the apple in this example is 0.5 grams per cubic centimeter, because \(\frac{100\text{ grams}}{200\text{ cm}^3} = 0.5\) grams per cubic centimeter. For the baseball, the density is \(\frac{145\text{ grams}}{200\text{ cm}^3} = 0.725\) grams per cubic centimeter.
In general, to find the density of an object, divide its mass by its volume.